On April 20, the GLSA and FOCM held their monthly online networking event. We always start the meeting with a featured presenter spending 10 – 15 minutes presenting information about themselves and a clinical research topic of relevance to them or to the industry.
This event featured Amy Baxter, MD; CEO and Founder of Pain Care Labs. PCL’s NIH-funded pain relief device is an ingenious intervention to reduce needle pain and fear. https://paincarelabs.com/
Amy presented information on research results showing the incidence of needle fear among Americans. Research shows that 25% of Americans are adverse to needles. This is playing a role in the slower than desired vaccination rates against COVID-19.
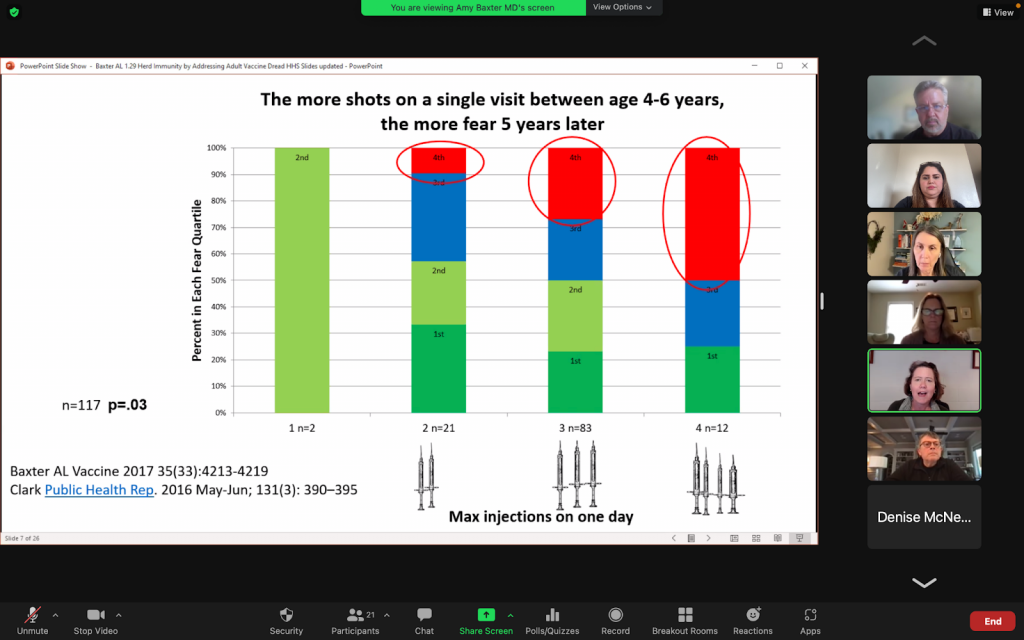
Needle fear has increased dramatically since 1995 when it was reported that 10% of adults and 25% of children feared needles. Prior to 1980, children’s last vaccination was at age 2, except for DPT boosters every 10 years. Since 1980, children receive booster injections between the ages of 4 and 6, when fears form. A study in 2012 from Canada shows 63% of people born after 2012 have needle phobia.
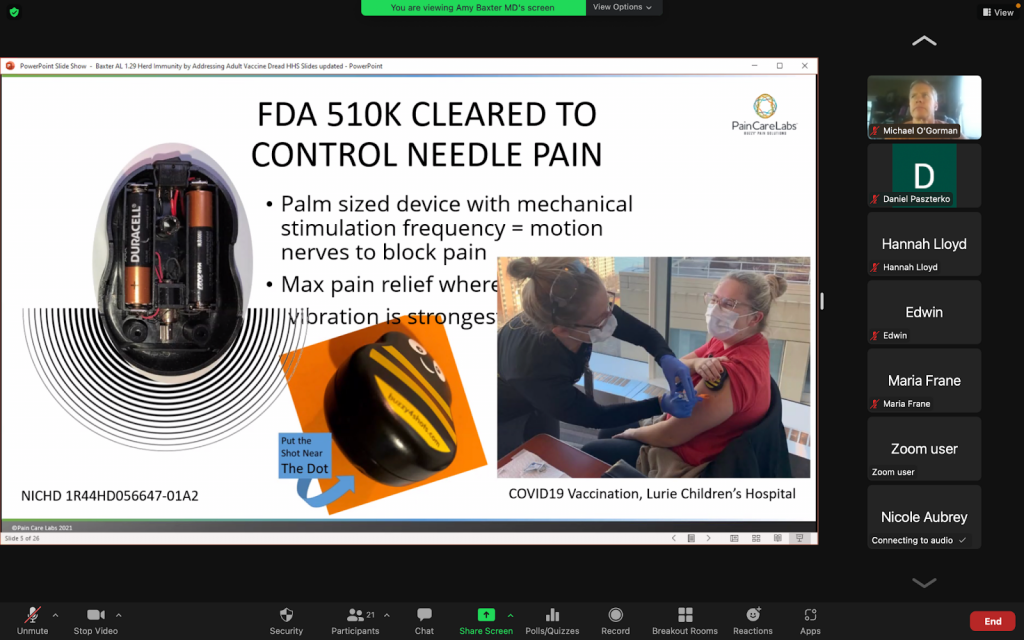
Pain Care Labs has developed Buzzy for the management of needle phobia. This combines the distraction of cold with deep vibration which blocks the pain signal. Managing needle phobia is important for increasing vaccination rates among people of all ages. It can also play a role in clinical research if a clinical trial is a vaccine trial or a protocol requiring multiple needle-sticks, Such trials may have higher drop-out rates or decline to enroll due to the subjects’ fear.
After the presentation, there were questions, answers and discussions. We then allowed newer participants the opportunity to tell us about their history in the industry and ask how the group can be of help. Additionally, after the event Heather Hollick shared observations about what makes the GLSA online networking events work so well is:
- Starting with initial casual conversation
- Having a 15-20 minute learning session
- Breaking into groups of 2-4 people several times so all can meet each other and share what they’re working on
- Ending with closing remarks, comments and observations.
- Online networking requires light moderation, because we’re missing body language for our cue as to who is to speak next.
Amy provided a good suggestion that we start these with a general ice breaker, a question or topic each can respond to. We’ll begin implementing that for our July event. The July and August events will be light-hearted and casual, imagine sipping a cocktail on a beachside outdoor deck with friends. In September, we’ll return to focused topics/presentations after the general ice breaker and before break out networking.
Attendees (bolded names indicate first time attendees):
Amy Baxter, MD; Pain Care Labs
Nicole O’Brien; Pain Care Labs
Peter Payne; Consultant
David Rodrin; IMA Clinical Research
Daniel Paszterko; Myonex
Ari Cofini; VeriSIM Life
Neil Banerjee; QMS Integrity
Nancy Zeleniak; Atrium Health
Mike Minor; IMA Clinical Research
Brittany Barber, Syneos Health
Nicole Aubrey; Aubrey Cole Consulting
Maria Frane, Simbec-Orion
Ravipal Luthra, University of Miami, Miller School of Medicine
Heather Hollick, Rizers LLC; Author of “Helpful, A guide to life, careers and the art of networking”
Mike O’Gorman, Life Science Marketplace
Edwin Gershom, Noble Life Sciences
Hannah Lloyd, Global Life Sciences Alliance
Denise McNerney, Global Life Sciences Alliance
Joe Buser, Global Life Sciences Alliance
Zulma Varela, Global Life Sciences Alliance
Sally Haller, Global Life Sciences Alliance
Screenshots from the event: