Fifteen people attended our May event.
We opened with input from others about recent attendance at a variety of conferences. Whereas the first quarter of the year had conferences exceeding typical attendance and a buzz of activity, the 2nd quarter seemed flat, with typical attendance and the feeling of a lull in activity. As discussed in one of our events this past fall, so much uncertainty in the world causes people to be conservative and hold back in spending money and starting projects. Those factors are:
Israel – Hamas war
Ukraine – Russia war
US Inflation
US Interest rate
US Presidential election
Predictions of recession
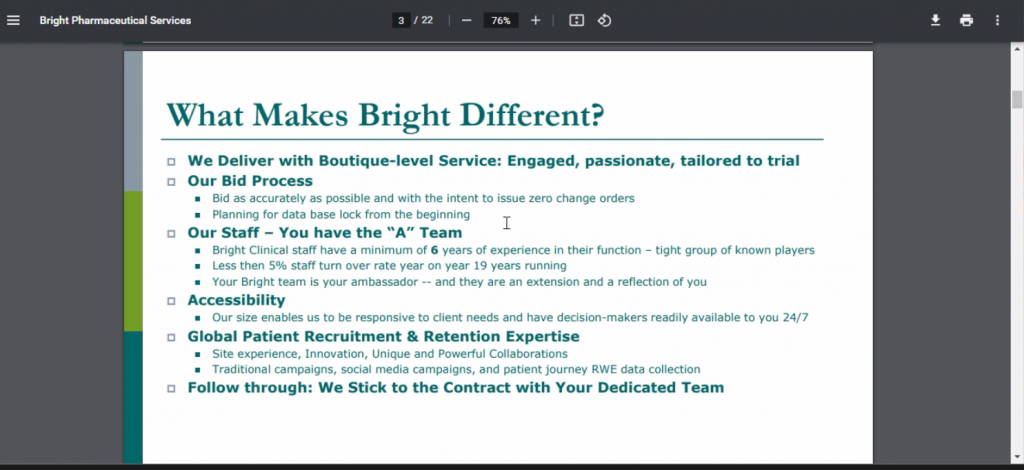
The presentation title was: Proactive vs Reactive Cryotank Management and Will Baird, a co-founder at Boreas Monitoring was the presenter. Boreas was founded in Wilmington, NC. Chris Matheus had seen Will present at an entrepreneurship/venture funding event at the Marine Biotech Center at UNC Wilmington in 2023.
Boreas was founded by lab professionals with over 80 years of industry experience owning/operating fertility laboratories. The CryoScout system was developed while the founders managed cryotanks, identifying inherent flaws in the temperature-based monitoring systems commonly used throughout the industry. It was accelerated by two high profile tank failures in 2018 that resulted in millions of dollars in lawsuits and catastrophic loss of patient samples, destroying people’s hopes & dreams of having children. They developed and patented a weight-based monitoring system — the most reliable method of determining the evaporation rate of a cryotank. It can detect a failure up to 84 hours earlier than temperature-based methods, giving labs a data-driven solution to monitor their tanks, along with enough time to save samples from being destroyed.
As a reminder, treat these events as an “open house” – drop in when you can and leave when you need to.
The link to get notifications of our future online and live events is: https://bit.ly/3UTb8hL
As our passion is to connect people and companies we know, like and respect to other people and companies we know, like and respect we always have everyone put the link to their LinkedIn profile in the chat to allow for easy connecting and future follow up.
GLSA’s LinkedIn page can be reached here: http://www.linkedin.com/company/global-life-sciences-alliance/
A reminder that Global Life Sciences has a select network of 50 clinical research service providers and we want to be seen as a resource to you and to the industry. So when you run into an issue or have a need, give us a call. It’s what motivates us, we quickly reach out to our contacts to find the solution you’re looking for.
The next event will be on June 12, a week earlier than normal due to the DIA Annual Meeting. The focus will be on a review of the first half of the year – comparing to last year and seeing what the sense of the industry is: trends, changes, etc.
ATTENDEES:
Will Baird, Boreas Monitoring
Jeanne Schow, Bleu Sales Leadership
Michael Young, biomedwoRx
Steve Sisson, MLM Medical Lab
Allie Ash, Colorado State University
Brandon Cantwell, Clinical Project Manager
Cameron Robinson II, Mustard Seed PMO
Eliana Rivera Burke, GreenLight Clinical
Joan Chambers, Tufts
Karen Tkaczyk, Vistatec
Lakshmi Ethirajan, Smabio
GLSA
Denise McNerney
Chris Matheus
Alex Hoppe